
Empowerly is a patient-centric platform custom tailored to improve the patient experience before, during, and after clinical trial participation. The platform gives participants connection to the study, provides clear communication on what to expect, and shows appreciation for their participation.
Sponsors are also able to get near real-time feedback from participants to learn about potential challenges that can be addressed before participants want to withdraw from a study. This feedback is critical especially for long-term follow up studies for cell and gene therapy where retention for up to 15 years is essential to understand durability of benefits and potential delayed or unforeseen risks.
This bi-directional engagement helps people feel like active partners in advancing research and increases the likelihood of feeling motivated to stay in trials, especially Long Term Follow-Up studies.
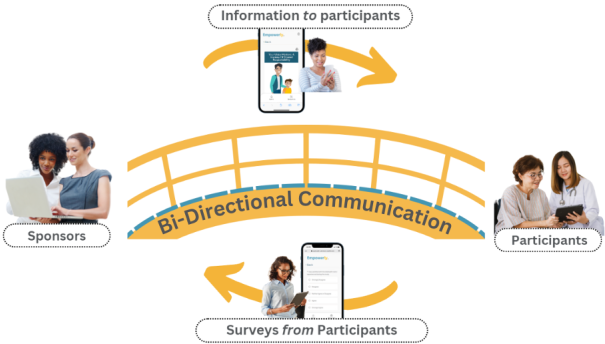
We Empower Bi-Directional Communication Before, During, and After Clinical Trials
Before
- Outgoing Content
Clear, inclusive communication to engage, educate, and empower potential participants.
- Incoming Questionnaire Responses
Pre-screening for trial eligibility based on self-reported data.
- Meeting people where they are
- Local and online communities
- Satellite HCPs and sites
- Patient organizations
During
- Outgoing Content
Just-in-time visit reminders, study resources, welcome, and thank you, high-level updates.
- Incoming Patient Experience Feedback
Customized Study Participant Feedback Questionnaire (“SPFQ”) cross-industry-developed patient experience surveys to capture insights.
- Continuous Engagement
Gratitude expressions from sponsor to participants.
After
- Outgoing Content
Return of research results (individual and aggregate) and updates on market approval status.
- Incoming Continued Engagement
- Long Term Follow-Up studies
- Real-world studies
- PRO development
- Informal surveys (e.g., ways to decrease trial burden)

- Personalizes digital experience to participant and care partner
- Delivers communication that participants want, when they need it, and to their preferred devices
- Reduces stress and confusion by providing clinical trial information in clear and inclusive language.
- Includes on-demand resource library for study-related content

- Aligns participant and site expectations for each site visit
- Provides explanatory overviews and rationale in advance of site visits
- Eases the burden to locate and distribute participant information
- Bypasses crowded communication channels such as emails and phone calls
- Enhances participant relationships with sites by providing clear, inclusive information that anticipates what participants will want to know and understand.

- Reduces likelihood of protocol deviations
- Reduces participant dropout and improves adherence
- Provides sponsors with a digital channel to communicate with participants
- Facilitates a sense of partnership in advancing science and patient care
Enroll more. Enroll faster. Ensure the right patients arrive predictably (those who meet the eligibility criteria) at clinical trial sites
We demystify the process for patients and ensure that patients are educated and prepared before they arrive at sites
We help create a sense of partnership with patients before they arrive at sites and aid our clients, using the same platform, to support participants during and after the trial