What We Experienced Together
At the 5th Chief Patient Officer Summit in Boston, conference attendees participated in a unique and novel simulation that placed them in the shoes of clinical trial participants.
In the immersive experience, attendees walked through two very different clinical trial paths:
- The Traditional Trial Experience – In this path, conference attendees experienced what too many trial participants know: uncertainty, frustration, and the feeling of being left in the dark. Attendees received minimal communication, faced unexpectedly long visits without preparation, and had little sense that their effort and contribution was of value toward the overall trial.
- The “Inside the Loop” Experience – Here, attendees experienced a reimagined clinical trial journey featuring proactive communication, emotional support, and a meaningful feedback loop that kept them informed and engaged. Trial participants want to feel they are kept “in the loop”.
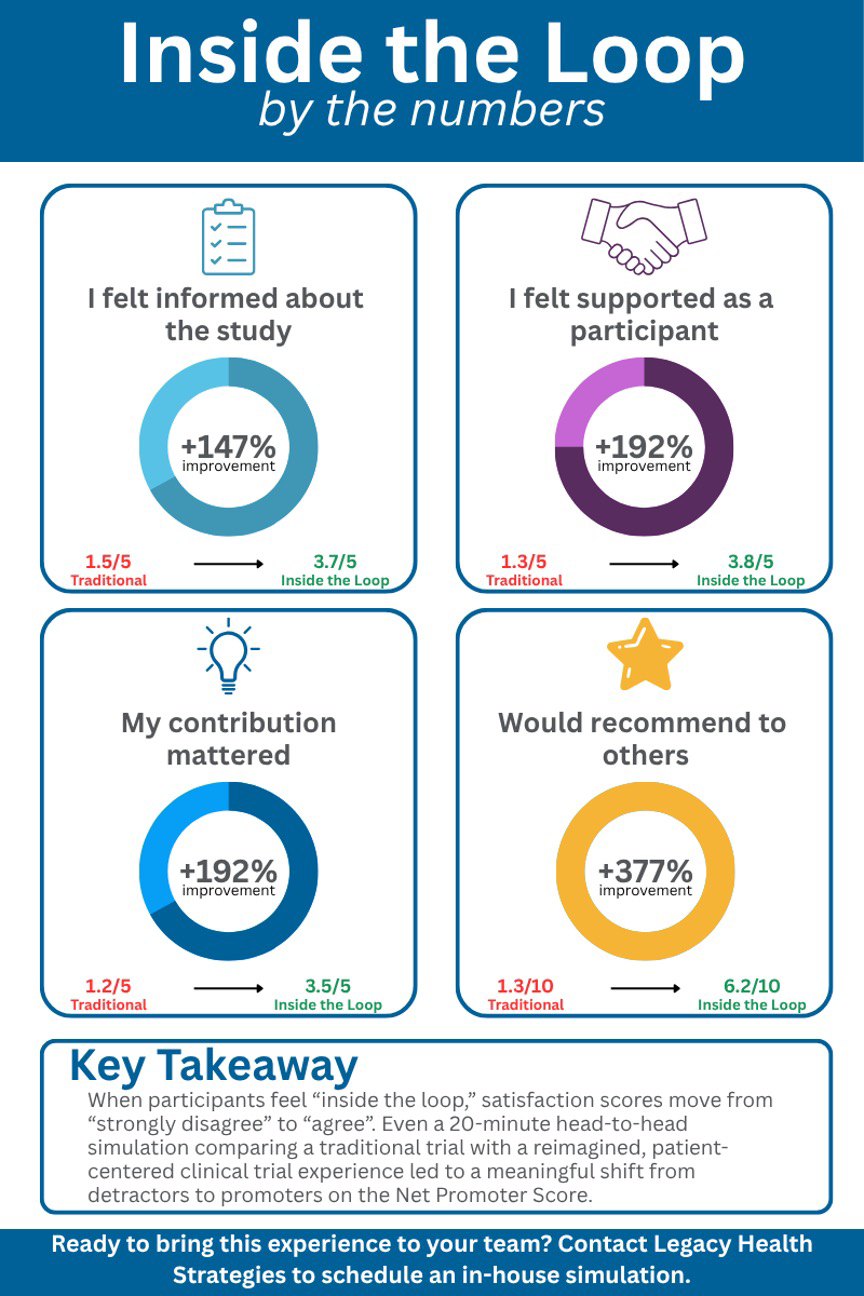
Attendees experienced both trial paths through a crossover design, starting with one, then switching to the other, to enable a direct, head-to-head comparison. Our hypothesis was simple: even in a 20-minute simulation, participants would feel a clear and meaningful difference between a “traditional” clinical trial experience and an “in the loop” experience. The results were striking. Bi-directional communication, enabled by a digital platform, fundamentally shifted the experience from one marked by uncertainty and burden to one grounded in clarity, connection, and shared purpose where each participant’s contribution felt seen, valued, and essential.
What the Data Show
The Inside the Loop Experience shifted participants from “strongly disagree” to “agree” on the 5-point Likert scales with “1” indicating strongly disagree and “5” strongly agree. Most remarkably, the Net Promoter Score moved participants from detractor territory to promoter range, turning potential critics into advocates.
What This Means for Sponsors
This brief immersive experience serves as a powerful proof of concept, one that Patient Engagement, Patient Advocacy, and Patient Recruitment and Retention teams can utilize for experiential learning within their organizations. In just an hour, cross-functional teams and senior leaders can feel the difference that bi-directional communication makes, deepening their understanding of what is possible before, during, and after a clinical trial.
Beyond the Numbers: The Human Impact
While the quantitative data is compelling, qualitative responses during the exercise revealed the deeper human impact of these two approaches:
- “It was a night and day experience. In one you just felt like you were a cog in a machine and in the second you felt like you were part of a solution.”
- “It was also the language difference. . . . regulation oriented versus person oriented”
- “This [Inside the Loop] feels supported and this [traditional model] felt like you are on your own.”
- “Even though no one was physically there, it felt like somebody was there. The [CMO] video and the questionnaire just felt like you were guided through.”
- “Seeing a human face . . . to have somebody who you can associate, especially someone who’s come from the sponsor makes you feel like it’s not this faceless entity.”
From Simulation to Reality
The contrast you experienced isn’t theoretical; it reflects a real and pressing decision point for every sponsor: whether and how we choose to communicate with participants. The “Inside the Loop” approach demonstrates what’s possible when we move beyond transactions to build true connection:
- Foster a sense of partnership and trust through timely, personalized communication and clear, patient-friendly updates
- Offer emotional support with guided resources and practical tools that participants can rely on throughout their journey
- Establish feedback loops that matter, ensuring participants feel genuinely heard, respected, and informed
- Honor their contribution by expressing appreciation for the time, contribution, and commitment it takes to advance science
The Path Forward
The conference attendees’ experience reinforces a human-centered truth: staying in a trial shouldn’t feel like a struggle—it should feel like a supported choice, grounded in guidance, connection, and partnership. When participants feel “inside the loop,” retention improves, data quality is enhanced, and participants become advocates who champion studies to their peers and providers.
The Inside the Loop simulation was developed by Legacy Health Strategies to demonstrate the transformative power of bi-directional communication in clinical trials. For more information about implementing these strategies in for your trials, please reach out!