Are you ready to give trial participants what they really want?
Installment 3 of the Data Return series by Jessica Scott M.D., J.D. (Legacy), Jennifer Millman (Legacy), and Joe Kim (ProofPilot).
Data return is becoming essential to enhancing patient trust, engagement, and ethical transparency. With the right tools and patient-centered strategies, sponsors can now easily and responsibly share individual data and study results. This guide offers a roadmap to implementing an efficient, ethical data return process that meets participant needs.
Brought to you by:


Installment Three
Implementing a Solution for Returning Clinical Trial Data to Participants
In the first two segments of this series, we explored what patients want from clinical trials and the ethical imperatives of returning data. Now, it’s time to address the practicalities: how can sponsors actually implement a solution for returning data to participants?
The Good News: Leverage Existing Technology + Implement Patient-Centered Strategy
Fortunately, the technological capabilities now exist to facilitate the return of clinical trial data, significantly lowering the entry barrier for sponsors. There is no need to reinvent the wheel. With thoughtful strategy and the right tools, sponsors can responsibly and ethically return the data that participants want to see.
The first step is simply deciding how you will start.
Below is a sample process map that details the key activities and components for the return of data to a clinical trial participant. At each step, we will provide topics for consideration to help you on your way.
The map is just one example of a good place to start intended to give you a sense of how to return participant data and aggregate results as plain language summaries directly to trial participants after a trial has ended without relying on trial sites.
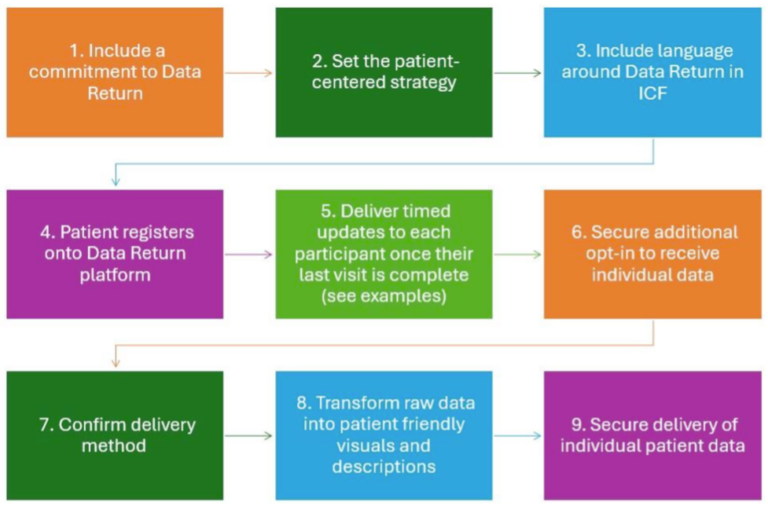
- Include a commitment to Data Return
- Start. Whether you begin with an enterprise-wide commitment at the policy level or choose to start with a pilot project, getting started somewhere is the key.
- Enterprise-wide rollout. With an enterprise-wide commitment, you could decide that as of a specific date, every new trial will include a plan for returning data and results. For example, starting on January 1, 2025, all Phase 2 and 3 trials in the U.S. and EU will include plain language summaries (PLS) sent directly to trial participants through a neutral third-party digital platform. You may also decide to include the study arm assignments, and you can ramp up from there.
- Pilot project. Starting with one or more pilot projects may help you to gather insights to inform a broader implementation. This approach allows you to manage complexities and refine before scaling up.
- Consider taking a risk-based approach. What is the population that could benefit the most from access to data?
- Permission to think big. In this scenario, we are limiting the data return to post-trial participant data and plain language summary of aggregate results; but, it could easily be adapted for a more robust strategy of returning additional data including screen fail results and labs that do not interfere with the integrity of the trial. Whether you choose to start small or go big, read on for things to think about.
- Set the Patient-Centered Strategy
- Leverage Patient Engagement. Integrate patient, community, site, and care partner insights into your data return strategy and communication plan.
- Ensure transparency. Inform patients about what data you will be making available and explain why some data elements are returned while others are not. For example, exploratory endpoints without clear clinical significance.
- Include language around Data Return in ICF
- Streamline the Consent Process. Simplify your data-sharing consent process to minimize the likelihood of overwhelming patients, recognizing volume of information patients receive at this stage.
- Provide Choice. Ensure there is a mechanism for participants to choose whether they wish to receive their data and provide an option should patients change their minds at the end of the trial.
- Detail Data Elements. Clearly communicate the frequency, timing, and type of data to be returned with context to help patients understand their results.
- Patient registers onto Data Return platform
- Minimize Risk. Use external vendor partners with expertise in developing and implementing a data return program to guide you through the process.
- Automate Processes. Avoid manual processes to enable a robust system for larger-scale roll-out. You may also want to anticipate the extent to which trial site investigators can and want to be involved if you are sharing data during the course of the trial. When sharing individual or aggregate results after the trial has ended, sites will likely be unable to assist and a post-trial communication platform will be critical.
- Decrease Site Burden. Consider a digital communication platform that allows sponsors to return data in a consistent manner across sites without relying on sites to share the information and, to the extent possible, without the need for sponsor support.
- Deliver timed updates to each participant once their last visit is complete</strong
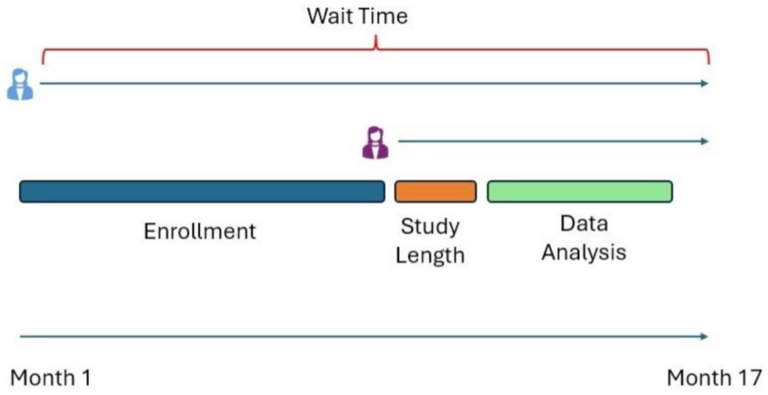
- Set expectations. Provide regular updates on data delivery timelines to manage participant expectations and build trust through clear and transparent communications. In developing the communications, it is important to keep in mind that the first patient last visit wait time will be a longer wait time than the last patient last visit.
- Bonus engagement. Note that regular updates provide a great way to keep your trial participants engaged during and after trial.
- Secure additional opt-in to receive individual data
- Logistic Planning. Provide an additional opportunity at the end of the trial for participants to opt-in or opt-out of receiving their data and results.
- Confirm delivery method
- Digital Platforms. Utilize a digital communication platform to handle data delivery, ensuring seamless and consistent communication even after sites close.
- Transform raw data into patient friendly visuals and descriptions
- Apply health literacy principles. Providing data without context can lead to confusion and anxiety. Ensure that all data and results are presented in plain language, avoiding technical jargon that may be difficult for participants to understand.
- Use visual aids. Incorporate charts, graphs, and infographics to help convey more complex information and ensure the information lands as intended by incorporating user-testing.
- Secure delivery of individual patient data
- Meaningful and Secure. Provide data in an easy to navigate, secure manner that protects patient privacy.
- Learn, adjust, ramp up. As we noted at the beginning, this sample process map focuses on returning data and plain language summaries at the end of the trial. As you gain experience and insights from your initial efforts, plan to scale up to an enterprise-wide program that provided consistent delivery across trials and participants. This will ensure fairness and equity to participants and avoid the perception of cherry-picking.

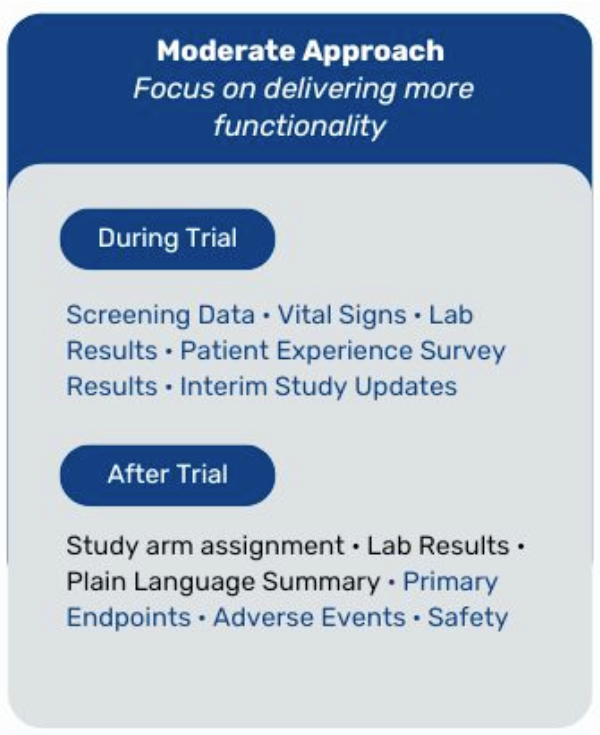
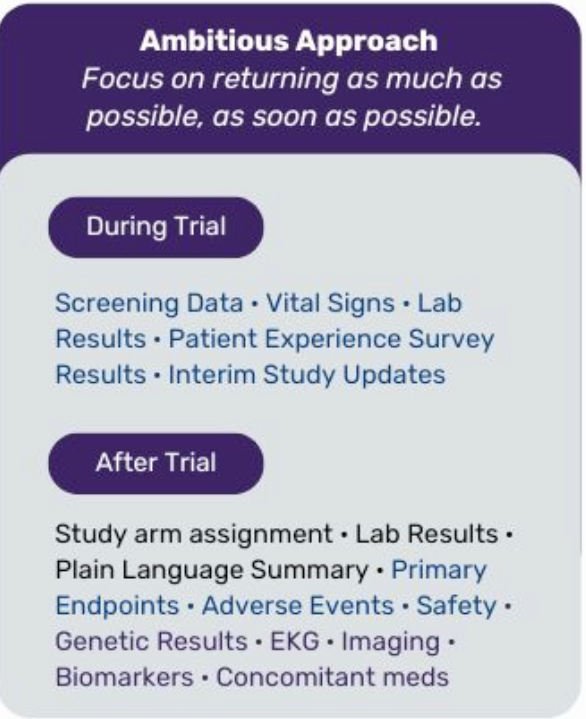
Final Thoughts
With the necessary guidance and practical models at hand, the bar to entry for returning participant data and results is more straightforward than ever. By making an enterprise-wide commitment or starting with pilot projects, sponsors can build trust, enhance transparency, and honor the contributions of clinical trial participants. The complexity of implementation is no longer an acceptable rationale for not moving forward. We are here to help organizations that are ready to deliver what patients really want, their data and results.
Prior articles in the data return series

