Jessica Scott, MD, JD presented on an innovation in Long-term Follow-Up for Cell and Gene Therapies at the 5th Chief Patient Officer Summit.
"Empowerly represents a forward-thinking approach that acknowledges a fundamental truth: to gather 15 years of crucial safety and efficacy data, we must first create an experience that participants want to stay engaged with for 15 years."
The Road Ahead: Revolutionizing Patient Engagement in Cell & Gene Therapy Long-Term Follow-Up Studies
Insights from Dr. Jessica Scott’s Presentation at the 5th Chief Patient Officer Summit West
The New Long-Term Follow-Up Frontier
The FDA projects 10-20 gene therapy approvals annually starting in 2025. Here’s the challenge: these groundbreaking treatments require long-term follow-up studies spanning 5, 10, or even 15 years. The stakes? Your long-term studies depend on engaged patients. Without strong retention, your data quality suffers – and so does your ability to demonstrate lasting safety and efficacy.
Cell and gene therapies, often referred to as advanced therapies, represent a paradigm shift in medicine, targeting the root causes of diseases and offering potential cures for previously untreatable, severe and life-threatening conditions. But their revolutionary nature comes with a critical requirement: sponsors must track patients for up to 15 years post-treatment to understand long-term safety and durability of beneficial effects.
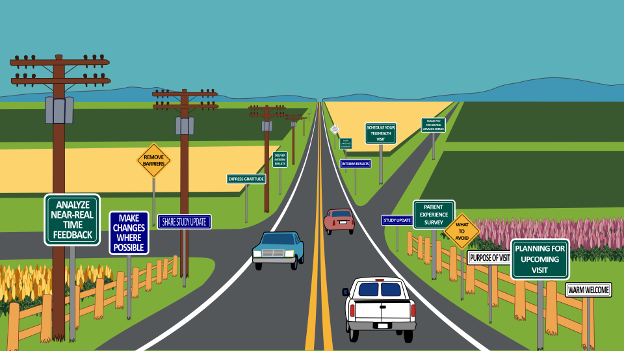
The Journey Becomes the Challenge
Imagine a highway stretching for many miles ahead. For patients in long-term follow-up studies, this highway represents their journey after receiving an advanced therapy in clinical trials or in the health care arena:
- With proper signage – clear, easy to understand, and placed well ahead of time – patients know what to expect and what to do.
- When the journey is smooth, patients stay on the road because they know it will take them where they need to go, experiencing a sense of purpose in their participation.
- Communication flows in both directions: outgoing information about upcoming study visits and procedures, and incoming survey feedback about the participant experience, life changes, and evolving roadblocks.
Now imagine driving on a road with confusing or limited signage, or worse – debris, rocks, and broken glass. At some point, drivers will want to exit, especially if their commitment to the destination wasn’t strong from the beginning.
This more challenging reality is what many patients face with LTFU studies. In fact, patients may drop out wanting to move on from visits to health care settings. After enduring extensive treatments, these patients may develop anxiety or avoidance behaviors that make continued participation emotionally taxing. Logistical burdens created by traditional study designs become increasingly difficult to manage over time. In-person visits, complex scheduling requirements, and travel to specific study sites, disrupt patients’ work, family responsibilities, and daily routines for years. As life circumstances change (relocations, new jobs, growing families), these practical challenges often become insurmountable.
Building a Better Highway with Empowerly
At the 5th Chief Patient Officer Summit West in San Diego (January 21-22, 2025), Dr. Jessica Scott presented an innovative solution to this critical challenge: Empowerly, a patient-centric platform custom-tailored to improve the patient experience.
Empowerly supports patients in long-term follow-up studies by:
- Creating Connection: Giving participants a sense of ongoing connectedness to the study and its importance
- Providing Clarity: Delivering clear communication about what to expect at each stage
- Showing Appreciation: Expressing gratitude and honoring the significant impact their long term participation has on advancing medical science and helping future patients.
- Gathering Participant Feedback: Collecting direct feedback through close-ended survey questions so sponsors can address issues before patients drop out
This two-way communication highway ensures participants feel the road was made with them in mind, proactively meeting their needs throughout their journey.
Why Patient Engagement Is Non-Negotiable
The success of long-term follow-up studies hinges on effective patient engagement strategies. Strong engagement:
- Enhances retention and compliance
- Improves data quality and completeness
- Builds trust and satisfaction among participants
- Makes studies more responsive to patient needs
- Supports regulatory requirements for extended safety and efficacy monitoring
Patient Engagement and Patient Advocacy functions within organizations can serve as strategic partners to help ensure quality long-term safety and efficacy data is provided to regulatory authorities – or risk withdrawal of these groundbreaking products from the market.
The Road Ahead
As we stand at the threshold of a new era in medicine, with 10-20 new cell and gene therapy approvals expected annually beginning this year, the question becomes not just how we develop these revolutionary treatments, but how we maintain the participant engagement to understand the longer-term outcomes of these advanced therapies.
Empowerly represents a forward-thinking approach that acknowledges a fundamental truth: to gather 15 years of crucial safety and efficacy data, we must first create an experience that participants want to stay engaged with for 15 years.
To learn more about Empowerly and how it can transform your approach to long-term follow-up studies and protect your therapy’s future, contact us today. More information on our website or email us for more information.